
Manufacturer: Nitta Gelatin Co., Ltd.
Storage conditions: Cold place (25 ℃ or less)
CAS RN ® : 9000-70-8 Molecular weight: 0
MedGel® Ⅱ is an improvement on the original MedGel® ("TaBaTa Gel for controlled release" outside of Japan),
a bioabsorbable gelatin hydrogel for the sustained release of physiologically active substances.
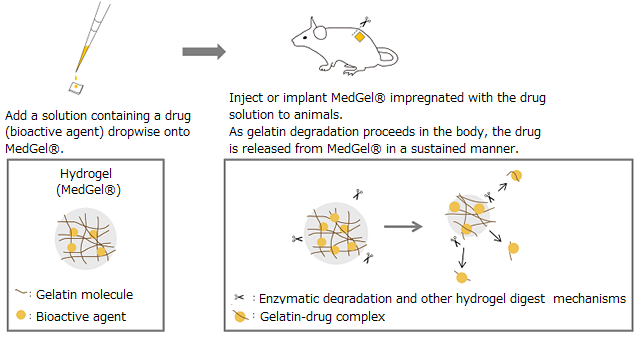
MedGel® ("TaBaTa Gel for controlled release" outside of Japan) is a bioabsorbable gelatin based hydrogel that can release physiologically active substances in a gradual and continuous manner. It was developed based on research by Prof. Yasuhiko Tabata of the Institute for Frontier Medical Sciences at Kyoto University. MedGel® is a water-insoluble polymer network formedby crosslinking gelatin. This polymeric network captures therapeutic agents primarily via electrostatic interactions. Following in vivo implantation MedGel® - therapeutic reagent complex, MedGel® is degraded by endogenous enzymes, allowing entrapped drugs to diffuse out of the network. MedGel® is also designed to be completely degraded and absorbed in the body. This eliminates the need for implant removal surgery after the drugs are exhausted.


[Sheet Type]
Nitta Gelatin Inc. offers two products (PI5 and PI9) with different isoelectric points. This enables you to select the hydrogel suitable for the isoelectric point (PI) property of your drug compound.
[Particle Type]
This form of hydrogel can be delivered via injection with a needle and syringe.
This product is a DDS base material for sustained release of gelatin-based physiologically active substances developed based on the research results of Prof. Yasuhiko Tabata, Research Institute for Virus and Regenerative Medicine, Kyoto University. This base material is made of gelatin cross-linked and insolubilized in water, and retains physiologically active substances through intermolecular interactions such as electrostatic interaction with gelatin. When implanted in a living body, the base material is degraded by a degrading enzyme such as collagenase secreted from tissue cells, and a physiologically active substance is gradually released as Medgel R is degraded. Medgel® is completely decomposed and absorbed in the body, so there is no need to remove it after the sustained release. There are two types of Medgel RII (PI5) and (PI9), each of which has a different type of gelatin. Due to the nature of the raw material gelatin, (PI5) is suitable for the sustained release of those that have a positive charge in a neutral solution, and (PI9) that has a negative charge in a neutral solution. However, it is known that the molecular weight and three-dimensional structure of bioactive substances also affect the intermolecular interaction between gelatin and bioactive substances. Please select the gel.